Chinese Scientists Deciphered the Origins of Repopulated Microglia in the Brain and Retina
Date:26-02-2018 | 【Print】 【close】
The regenerative capability of the central nervous system (CNS) is largely limited due to the intrinsic properties and external environments. Once the brain gets injured, it is impossible to repair and restore the tissue to the normal situation. However, this notion has been challenged by a recent study.
Microglia are the long-lived myeloid cells in the central nervous system (CNS). Elmore and Green et al reported a newly-discovered microglial progenitor cells in Neuron at 2014, this finding for the first time showed the microglial progenitors in the adult brain, providing potential insights on regenerative medicine.
However, the origin of repopulated microglia is still hotly debated, the source of repopulated microglia remains highly controversial.
Recently, a research paper entitledRepopulated microglia are solely derived from the proliferation of residual microglia after acute depletion has been online published in Nature Neuroscience from PENG Bo Lab at Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences. In this paper, Peng and his colleagues successfully deciphered the origin of repopulated microglia in the brain by a series of fate mapping approaches.
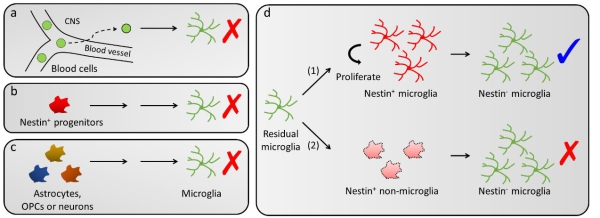
The authors first excluded the blood origin of repopulated microglia via parabiosis, a surgical approaches generating chimeric mouse of exchanged blood cells (Figure 1a). Strikingly, they then demonstrated repopulated microglia were NOT differentiated from Nestin-positive progenitor cells (Figure 1b). The authors also demonstrated astrocytes, oligodendrocyte precursor cells (OPCs) or neurons were not the precursor cells of repopulated microglia (Figure 1c). In contrast, the authors demonstrated that ALL repopulated microglia in the brain were derived from the proliferation of few microglia surviving on pharmacological ablation (<1%), and the dividing microglia transiently expressed Nestin (Figure 1d). The results thus provided solid evidences that repopulated microglia were solely derived from residual microglia rather than de novo progenitors, indicating the absence of microglial progenitor cells in the adult brain. In addition, by RNA sequencing, the authors found that the repopulated microglia may share similar functions as the resident microglia in homeostatic and diseased brains.
Moreover, retina is an important part of the CNS. The capacity of cell regeneration is even more limited in the adult mammalian retina. Although a few cells are sporadically regenerated in the retina, no massive cell regeneration had been observed.
The paper entitled Dual extra-retinal origins of microglia in the model of retinal microglia repopulation in Cell Discovery, Researcher PENG Bo and his colleagues found that inhibition of CSF1R eliminated all resident microglia in the retina (100%) (Figure 2), different from 99% of the brain. If retina shares the similar mechanism of microglia repopulation as that of the brain (Figure 1), newborn microglia will not emerge and repopulate the retina since there are no survival microglia after depletion (Figure 2). However, the authors surprisingly found that new microglia emerged and rapidly repopulated the whole retina after removal of CSF1R inhibition (Figure 3). This is the first time observing the robust and massive cell regeneration in the adult mammalian retina. And the origin(s) of retinal microglia is a mystery.
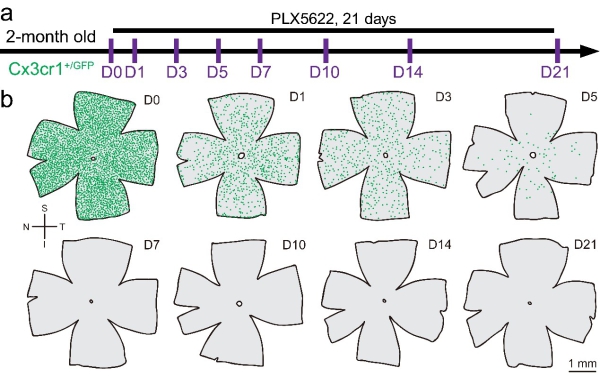
Figure 2. Inhibition of CSF1R by PLX5622 rapidly deplete all microglia in the retina.
(a) Scheme of PLX5622 administration and time points for observation.
(b) Spatial distributions of retinal microglia show that the cell numbers are reduced after PLX5622 administration. Each green dot represents a microglial cell.
S: superior; I: inferior; N: nasal; T: temporal; PLX5622: PLX5622 (a CSF1R inhibitor) formulated diet. (Image by PENG Bo)
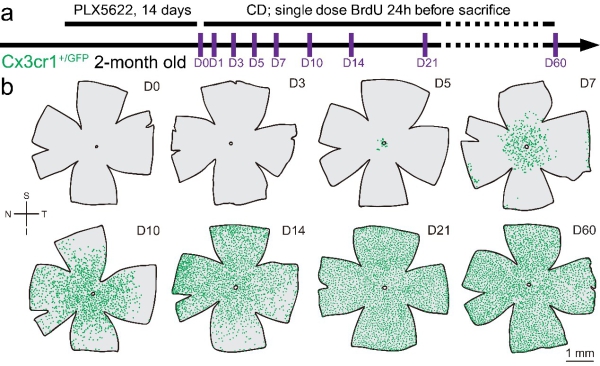
Figure 3. Repopulated microglia replenish the entire retina after removal of CSF1R inhibition.
(a) Scheme of microglial repopulation and time points for examination.
(b) Spatial distributions of retinal microglia show that microglia repopulate the whole retina after removal of PLX5622. Each green dot represents a microglial cell.
S: superior; I: inferior; N: nasal; T: temporal; PLX5622: PLX5622 formulated diet; CD: control diet. (Image by PENG Bo)
Then the authors investigated the origin(s) of repopulated retinal microglia and found that repopulated retinal microglia have two populations of distinct origins: the majority of center-emerging microglia and the minority of periphery-emerging microglia. The center-emerging microglia were solely derived from residual microglia in the optic nerve, whereas the periphery-emerging microglia were derived from macrophages in the ciliary body/iris (Figure 4). By using the microglial repopulation model, the authors thus for the first time observed a robust and massive cell regeneration in the adult mammalian retina. Furthermore, they first identified extra-retinal origins of microglia in the adult retina, shedding new light on the origins and maintenance of microglia in the retina.
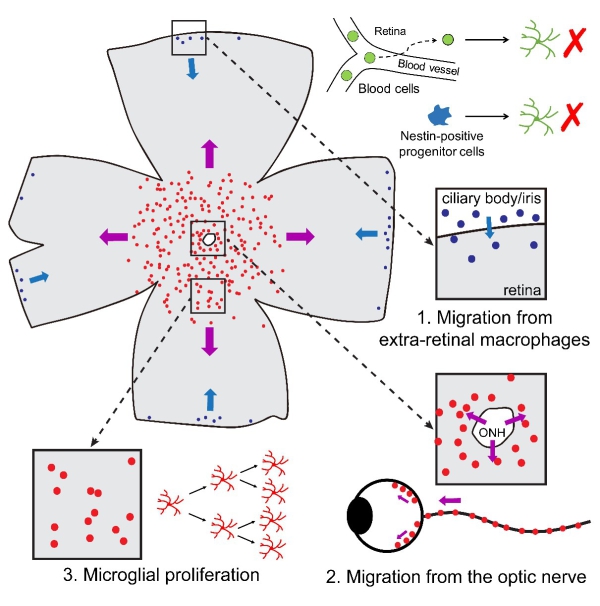
Figure 4. The origins of repopulated microglia in the retina. (Image by PENG Bo)
These studies were mainly conducted by PENG Bo Lab with collaboration of Dr. RAO Yanxia at the University of Hong Kong and Prof. YUAN Ti-Fei at Shanghai Jiao Tong University. Dr. HUANG Yubin and Dr. XU Zhen are the co-first authors of these two studies.