Scientists Develope “Trojan Horses” MRI Tracking for Glioma Therapy
Date:16-11-2018 | 【Print】 【close】
Cell-based drug delivery systems have been increasingly explored as “Trojan horses” to carry concealed drug cargoes for tumor-targeted therapy owing to the intrinsic tumor-homing and drug-carrying property of some living cells. However, imaging tracking of their migration and bio-effects is urgently needed for clinical application, especially for glioma.
A research team led by Prof. ZHENG Hairong at the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences, developed an intelligent biomimetic theranostic platform by integrating inflammation-activatable neutrophils with magnetic mesoporous silica nanoparticles (MMSNs) for treating remaining glioma after surgical resection of primary tumors.
Neutrophils, a type of polymorphonuclear leukocyte, play a critical role in immune responses. First, they can be activated within the vasculature and move along the chemotactic gradients towards the inflammatory sites, and eliminate the pathogens by phagocytosis.
Second, they possess the native ability of crossing BBB (blood brain barrier) /BTB (blood tumor barrier) and infiltrating the tumor mass. It is worth noting that neutrophils are phagocytic cells and can uptake various nanoparticles.
And third, neutrophils are terminal-differentiated cells with an average half-life of 6-7 hours, thus the magnetic resonance imaging (MRI) signal would not decrease due to cell proliferation and cellular exocytosis.
MMSNs combine the merits of magnetic Fe3O4 core that provides contrast enhancement for MRI, and mesoporous silica shell for the encapsulation/sustained release of chemotherapeutic agents. Doxorubicin (Dox), a model antitumor drug, was loaded into MMSNs (D-MMSNs) and coincubated with neutrophils from peripheral blood of health mice, thus producing an intelligent biomimetic theranostic platform ND-MMSN. The phagocytized D-MMSNs possess high drug loading efficiency and do not affect the host neutrophils’ viability.
After systemic injection of ND-MMSNs into the inflamed mouse glioma model established by surgically resecting part of primary glioma, they can migrate along the molecular guidance signals, emigrate outside the vasculature and accumulate in the inflamed glioma sites, resulting in the inflammation-triggered neutrophil recruitment. Subsequently, highly activated neutrophils carrying D-MMSNs release neutrophil extracellular traps (NETs) in the inflammatory region. The concomitant release of D-MMSNs cargos are uptaken by infiltrating glioma cells, achieving precise diagnosis and high anti-glioma efficacy.
Moreover, the phagocytic MMSNs possessed cell tracking capability, providing the potential for the diagnosis of residual tumor and therapeutic guidance. Importantly, improved survival rate and delayed glioma relapse, which were demonstrated in the surgically treated glioma mouse models after treatment with ND-MMSNs, suggesting that neutrophils carrying D-MMSNs could effectively identify the inflammatory signals derived from surgical management and accumulate in the remaining tumor site to maximize the drug bioavailability.
This strategy provides a new insight to track the fate of neutrophils by MRI and explore immune cell-based drug delivery systems for treating diseases associated with inflammation.
The study entitled “MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma" was published in Nature Communications.
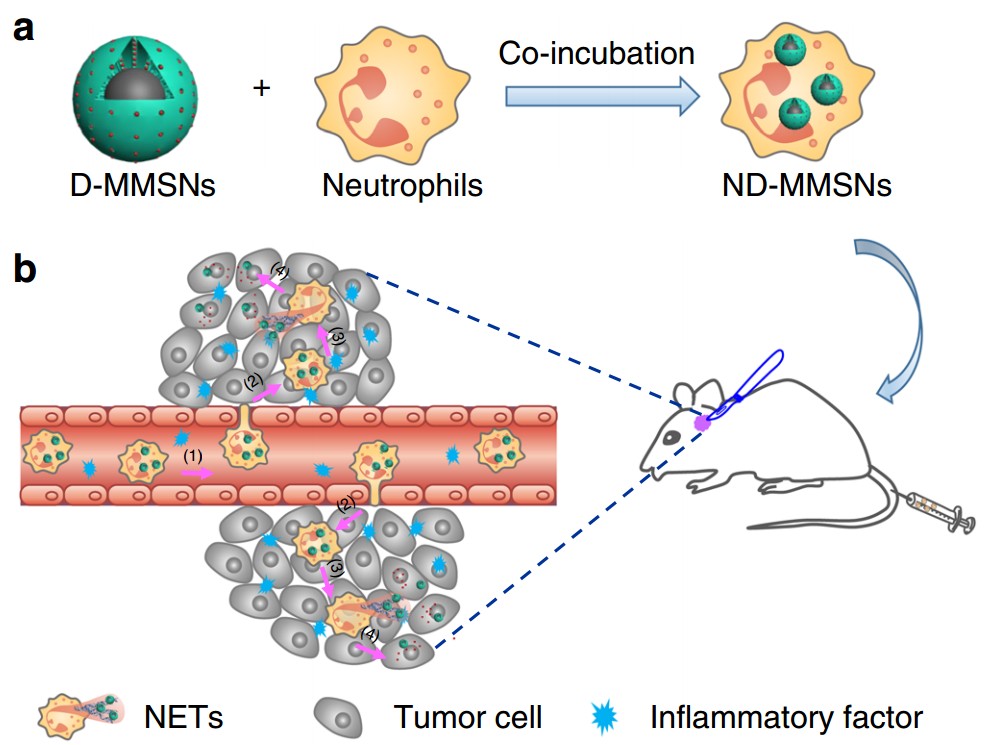
Fig. Fabrication and targeted-therapeutic schematics of ND-MMSNs. (a) Schematic illustration of the preparation of ND-MMSNs. (b) Schematic shows that inflammation-activatable ND-MMSNs target inflamed glioma sites and phagocytized D-MMSNs would be released to achieve residual tumor theranostics.