An Effective Feedstock for the Synthesis of Phosphorus-Based Chemicals
Date:18-06-2019 | 【Print】 【close】
Phosphorus-based chemicals are widely used as flame retardants, agrochemicals, battery electrolytes, catalyst ligands, and pharmaceuticals. Most phosphorus-based Industrial production chemicals is based on white phosphorus (P4).
However, the P4-based synthetic methods generally involve multiple steps and toxic materials. Therefore, it is of both scientific and commercial interest to identify alternative synthetic techniques to directly convert low-toxic materials into valuable phosphorus containing compounds.
In response to this challenge, a research team led by Prof. YU Xuefeng at the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences developed novel concept of synthesizing organophosphorus compounds directly from black phosphorus (BP) nanoparticles as the feedstock. Their findings were published in CCS Chemistry.
BP is a shining star among various types of 2D materials, due to its distinctive properties. The team has been devoted to researching the chemical activity of BP since 2016. The studies, stability of BP under ambient conditions, reduction of BP and nucleophilicity of BP have been revealed.
“The high chemical activity of BP suggests that it can react with organic substrates to form multiple phosphorus containing compounds.” said Prof. YU.
In this work, the team used BP for efficient synthesis of organophosphorus compounds. Compounds such as alkyl phosphines, alkyl phosphine oxides, phosphine sulfide, and hexafluorophosphate anion are prepared with good isolation yields under mild conditions. Selective synthesis of primary, secondary, and tertiary organophosphorus compounds is also demonstrated utilizing this one-pot approach.
"Compared with traditional white phosphorus (P4)-based methods, the new synthetic concept and process utilizing elemental phosphorus are more efficient and environmentally friendly. " said Prof. YU, “We hope this process will spur industrial production of phosphorus compounds.”
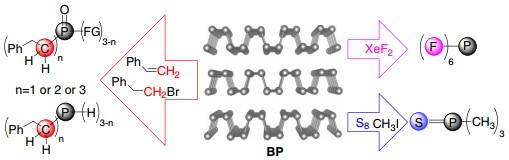
Figure. Chemical reactivity of BP toward formation of new bonds with carbon, sulfur, and fluorine. (Credit: Prof. YU Xuefeng)
CCS Chemistry is the Chinese Chemical Society’s flagship publication, established to serve as the preeminent international chemistry journal published in China. CCS Chemistry will have global reach, both in terms of contributions and readership. All articles are available as Open Access immediately upon publication at no cost to contributing authors. SIAT is a member of the Governing Body.
CONTACT:
ZHANG Xiaomin
Email: xm.zhang@siat.ac.cn
Tel: 86-755-86585299