Magnesium-based Dual-ion Batteries:
Use Insoluble Organic Anodes in an Innovative Way
Date:13-05-2020 | 【Print】 【close】
Lithium ion batteries (LIBs) are currently one of the most commonly used batteries, but suffer from several limitations, its low abundance does not make it ideal to be applied in large-scale energy storage.
Instead, Magnesium-ion batteries (MIBs) shows good application prospects in the field of large-scale energy storage since their superiority of high capacity and abundant reserves, which it’s a promising substitute of LIBs.
However, magnesium metal anodes are prone to form passivation layer in organic electrolytes, triggers irreversible platting/stripping of magnesium ions. In addition, the absence of athode materials that reversibly store magnesium ions, results in the restriction the development of MIBs.
Compared with traditional inorganic electrode materials, organic electrode materials exhibit excellent competence for magnesium ions storage due to the mild redox reaction between their functional groups and ions.
At the same time, if the working mechanism of the dual ion battery can be combined, the high reaction potential (> 4.5V vs Li+/Li) and fast anion diffusion kinetics of the anion intercalated graphite cathode will tremendously improve the working voltage and electrochemical performance of MIBs.
Based on the above research situation, A research team led by Prof. TANG Yongbing from the Shenzhen Institutes of Advanced Technology (SIAT), Chinese Academy of Sciences, has successfully developed a magnesium-based dual-ion battery (Mg-DIB) based on insoluble organic anode materials.
In this study, Mg-DIB was fabricated by using 3, 4, 9, 10-perylenetetracarboxylic diimide (PTCDI) as organic anode, expanded graphite (EG) with high potential and fast anion diffusion kinetics as cathode, ionic liquid as electrolyte.
The Mg-DIB delivered extraordinary rate performance and cycle performance, with 85% capacity retention at high current density of 20C (1C=100 mA g-1) and 95.7% capacity retention after 500 cycles at 5C.
Through the combination of the in-situ fourier transform infrared (FTIR) spectroscopy, theoretical calculation analysis and other characterization methods, it confirmed that there were the threefold coordination mechanism and hydrogen bond formation during Mg-ion insertion into PTCDI, which contributed to good insolubility in organic electrolytes and good structural stability. Furthermore, the insolubility played an important role on improving the cycling stability of Mg-DIB.
"By reasonably designing and engineering the functional group structure of small-molecule organic materials magnesium ion battery structure system, a wide range of insoluble organic materials can be available. Simultaneously, taking into account the advantages of dual ion battery system, the research of Mg-DIB will open up a new way to design high-performance MIBs and other energy storage devices,”Prof. TANG said.
The study has been published online in the journal of Energy Storage Materials on April 28, a prestigious online journal in the field of new energy and materials science.
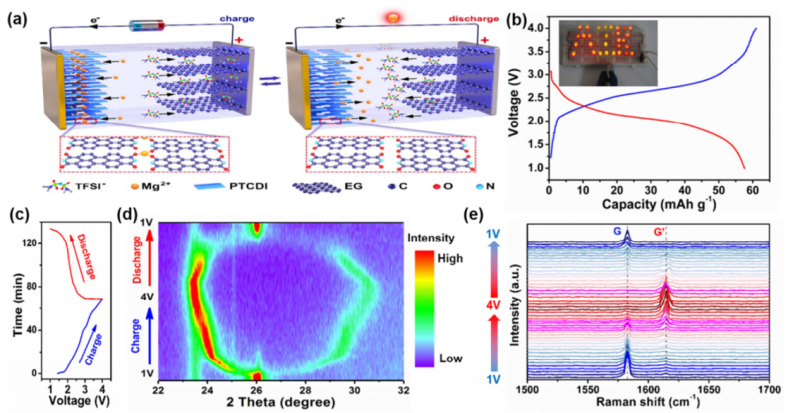
Figure. (a) Schematic illustration of the working mechanism of Mg-DIB based on the PTCDI anode and EG cathode. (b) Typical galvanostatic charge/discharge curves of the Mg-DIB at 2C in the voltage range of 1-4 V. Inset photograph is one Mg-DIB coin cell that can light up 29 LEDs in parallel. (c) Charge/discharge voltage profiles of the Mg-DIB at 2C. (d,e) In-situ XRD patterns (d) and in-situ Raman spectra (e) of the EG cathode in the Mg-DIB under different charging/discharging state. (Image by LEI Xin)
ZHANG Xiaomin
Email: xm.zhang@siat.ac.cn