Subtype-specificity in Sleep Regulation in Drosophila
Date:06-02-2023 | 【Print】 【close】
The coherence of regulation of sleep by different brain regions and nuclei in mammals is complex. The ellipsoid body (EB), as a major structure of the central complex of the Drosophila melanogaster brain, is relatively simple compared to mammals, but also exhibits a high level of connectivity and functional heterogeneity while tuning multiple behaviours in real-time, including sleep.
Twenty-two subtypes of EB ring neurons have been identified based on anatomical and morphological characteristics by light-level microscopy and EM connectomics. Limited studies have associated ring neurons with the regulation of sleep homeostasis and structure. But, in spite of these recent progress, the cell-type specificity of sleep regulation is largely unknown.
Prof. LIU Chang’s team from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences (CAS) in collaboration with Prof. Leslie C. Griffith’s team from Brandeis University, USA, focused on advancing the unknown subtype-specificity of sleep regulation.
Lately, they published a study entitled "Subtype-specific roles of ellipsoid body ring neurons in sleep regulation in Drosophila" in the Journal of Neuroscience as the cover/featured article, the team recruited an unbiased interrogation of the roles of subtypes of ring neurons in sleep regulation, marrying experimental data with mathematical models to understand distinct aspects of sleep in the fly.
From a thermogenetic screen of EB drivers that label multiple subtypes of EB ring neurons, researchers provided an overview of how multiple ring neurons are involved in the modulation of amount of sleep, sleep structure and their effects on sleep pressure and/or depth upon activation of these neurons. In addition, analysed of data for probability of transition from awake to sleep or from sleep to awake using a mixed Gaussian model detected 5 clusters of GAL4 drivers which had similar effects on sleep pressure and/or depth during the day and/or night, respectively.
Lines driving arousal contained R4m neurons, whereas lines that increased sleep pressure had R3m cells. Furthermore, a general linear model analysis correlating ring cell subtype and activity-dependent changes in sleep parameters across all lines identified several cell types significantly associated with specific sleep effects: R3p was daytime sleep-promoting, and R4m was night time wake-promoting. Finally, using genetic suppression of intersected population strategy, a subpopulation of neurons which exclusively affect sleep structure, R3d cells, were identified to contribute to fragmentation of sleep during both day and night.
"Taken together, multiple subtypes of ring neurons distinctively control sleep amount and/or structure," said Prof LIU, "the unique highly interconnected structure of the EB suggested a local-network model worth future investigation, and we believe these findings will aid in revealing the principles of integration and cooperation in the brain."
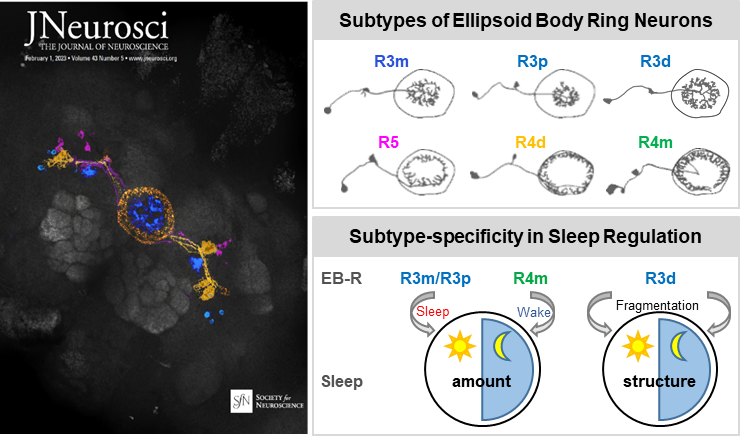
Left: Cover image. Right: Schematic of sleep/structure regulation by multiple subtypes of ring neurons. (Image by Prof. LIU)
Media Contact:
ZHANG Xiaomin
Email:xm.zhang@siat.ac.cn