Impaired Inheritance of Parental Histones Accelerates Breast Cancer Growth and Metastasis
Date:14-06-2023 | 【Print】 【close】
Chromatin states and their associated epigenetic information play a crucial role in maintaining the identity of cells as they divide. Histone post-translational modifications (PTMs) are important determinants of cellular epigenetic state, carrying epigenetic information and regulating gene transcription.
Epigenetic aberrations are linked to various diseases, including cancer. However, the role of parental histone inheritance in tumorigenesis or tumor evolution remains unclear.
A team from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences has now developed a tumor model to investigate impaired inheritance of parental histones. They introduced an MCM2 histone-binding domain (HBD) mutation in breast cancer cell lines. In this model, impaired histone inheritance leads to significant epigenetic reprogramming, particularly affecting the repressive histone mark H3K27me3, thereby promoting tumor growth and metastasis.
The study published in Nature Communications on June 10, explored the impact of impaired parental histone inheritance on histone modification profiles in MCM2 mutant cells. The researchers observed changes in the distribution of multiple PTMs, including both repressed and active histone marks. The loss of H3K27me3 at the promoters of development-related genes resulted in their activation in cancer cells.
Furthermore, cancer cells with impaired histone inheritance exhibited accelerated growth and a tendency towards increased aggressiveness after orthotopic transplantation. Subsequent single-cell RNA sequencing (scRNA-seq) analysis revealed that newly formed subclones in cancer cells with histone inheritance disorders promoted tumor progression. These subclones acquired advantages in proliferation and fitness, evolving faster when faced with more complex environments.
This study confirms the crucial role of parental histone inheritance carrying H3K27me3 in maintaining the specific regions of differentiated cells. Failure to restore H3K27me3 can reactivate mammary gland development processes that are often exploited by breast cancer cells as drivers of tumor progression.
"These findings provide valuable insights into how epigenetic instability contributes to tumor progression," said Professor GAN Yunhai, a corresponding author of this study, "suggesting that targeting abnormal epigenetic inheritance may improve patient outcomes by preserving epigenetic stability."
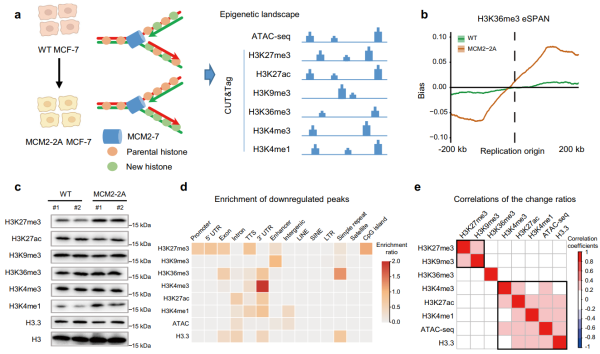
Impaired histone inheritance results in genome-wide epigenetic reprogramming in MCM2-2A mutant MCF-7 breast cancer cells. (Image by SIAT)
Media Contact: ZHANG Xiaomin
Email:xm.zhang@siat.ac.cn