Optimizing Cytochrome P450 Network for High Level Production of Quillaic Acid: the Aglycone of Natural Adjuvants
Date:20-10-2023 | 【Print】 【close】
The versatile role cytochrome P450s (CYPs) play in nature makes them an important tool in synthetic biology for building microbial cell factories (MCFs) to produce commercially valuable pharmaceuticals, such as saponins. However, CYPs can exhibit substrate promiscuity and oxidize unintended substrates, which lead to accumulation of under-oxidized or over-oxidized products not recognized by other co-expressed CYPs.
Quillaic acid (QA) is the aglycone backbone for 49 out of 58 saponins identified in Quillaja saponaria Molina (QsM), some of which have been used as adjuvants in malaria, shingles, and Covid-19 vaccines. De novo biosynthesis of QA in a MCF requires multiple CYPs to catalyze the six-step oxidation at three different positions. Maintaining the oxidation steps in absolute synergy is crucial, because 47 different products could be formed during the process, of which 46 are either incomplete or over oxidized by-products.
Recently, a research team led by Howard H. CHOU at Shenzhen Institute of Advanced Technology of Chinese Academy of Sciences has developed a combinatorial optimization approach to construct and spatially control a CYP-CPR network in an engineered Saccharomyces cerevisiae strain that favors QA production.
The study was published in Metabolic Engineering on Sep. 12.
In this study, an efficient network of CYPs and cytochrome P450 reductases (CPRs) was established through iterative screening of more than 100 CYP-CPR pairs to produce the target product QA.
Furthermore, the spatial distance between different CYPs on the endoplasmic reticulum was controlled by expression of membrane steroid-binding protein 1 from Arabidopsis thaliana, which increased QA titer by 79.5%.
Finally, the enzyme network in the MCF was optimized by strengthening co-factor supply and augmenting ER surface. Out of 47 potential products that could be synthesized, 86% of the products synthesized by the optimized enzyme network was the target compound QA, and fermentation achieved 2.23 g/L QA.
"Our work demonstrate the feasibility of using a combinatorial optimization strategy to assemble an enzyme network that catalyzes the multi-step oxidation of inert substrates to synthesize functional scaffolds for building pharmaceutically active molecules," said Prof. CHOU.
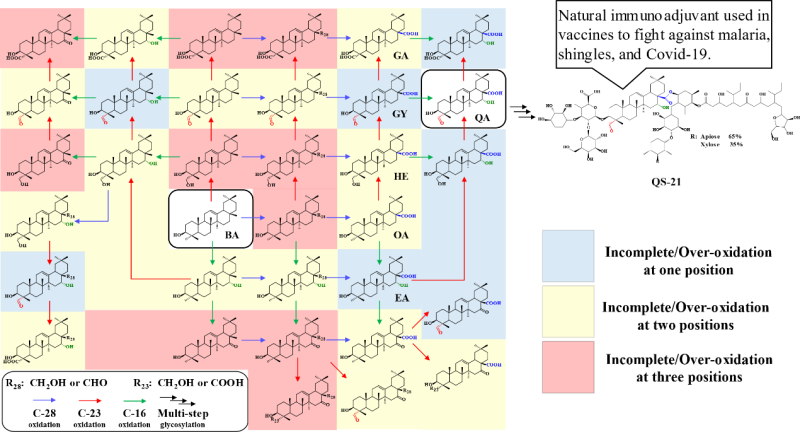
Potential products formed from expressing CYPs to oxidize β-amyrin to quillaic acid. (Image by SIAT)
Media Contact:
ZHANG Xiaomin
Email:xm.zhang@siat.ac.cn