Innovating Pathway Engineering at the Crossroads of IT and BT: A Novel Strategy for Overcoming Genetic Constraints
Date:28-02-2024 | 【Print】 【close】
Pathway engineering faces a critical challenge: genes' evolutionary potential and adaptability are limited by epistatic effects, causing uncertainty and sluggish evolution. For example, a small tweak in one enzyme may inadvertently bottleneck another enzyme in the pathway, delaying enhancement or the emergence of new functions for thousands of years. The goal is to replicate millennia of natural evolution in much shorter timeframes and with fewer iterations.
To address the above issue, a research team led by Prof. LUO Xiaozhou from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences and his international collaborator Prof. Jay D. Keasling constructed a biofoundry-assisted strategy for pathway bottlenecking and debottlenecking. This enabled the parallel evolution of all pathway enzymes along a predictable evolutionary trajectory. The study also incorporated the ProEnsemble machine learning framework, further alleviating the influence of epistatic effects among genes in the evolutionary pathway, thereby creating an efficient and universal flavonoid chassis.
This study was published in the Advanced Science titled "Pathway evolution through a bottlenecking-debottlenecking strategy and machine learning-aided flux balancing" on February 6.
The team unveiled a novel approach to pathway engineering, addressing the intricate challenges of enzyme evolution. This method, known as the bottlenecking and debottlenecking strategy, strategically guides enzyme evolution within heterologous pathways. By creating bottleneck points and introducing targeted mutations, enzymes are driven to adapt and evolve towards desired traits. Once achieved, debottlenecking steps restore pathway flow, enhancing performance. Crucially, this approach simplifies selection by using final product concentration as the sole criterion, streamlining experimentation.
Automation accelerates screening of genetic variants, while artificial intelligence algorithms analyze data to optimize pathway parameters. Together, these strategies offer a comprehensive framework for pathway optimization, opening new avenues in biotechnology and synthetic biology.
This research effectively tackles the technical hurdles linked to pathway evolution uncertainty. It marks another notable stride at the crossroads of IT (Information Technology) and BT (Bio-technology) by LUO's group, building upon their 2023 breakthrough with the UniKP large language model framework for enzyme mining and evolution (Nat. Commun. 2023).
Moreover, the study seamlessly integrates the automation and machine learning advantages, substantially accelerating chassis development and reducing economic overheads. This offers a pioneering technological blueprint and innovative approaches for propelling the field of bio-intelligent manufacturing forward.
"Heterologous pathway engineering has become a crucial aspect of biosynthesis," stated Prof. LOU. "We warmly welcome long-term postdoctoral researchers with backgrounds in biology, chemistry, bioinformatics, biomedical engineering, or expertise in enzyme-directed evolution, machine learning, high-throughput screening, and natural and synthetic compound biosynthesis. Together, we aspire to revolutionize the realms of synthetic biology and industrial biotechnology."
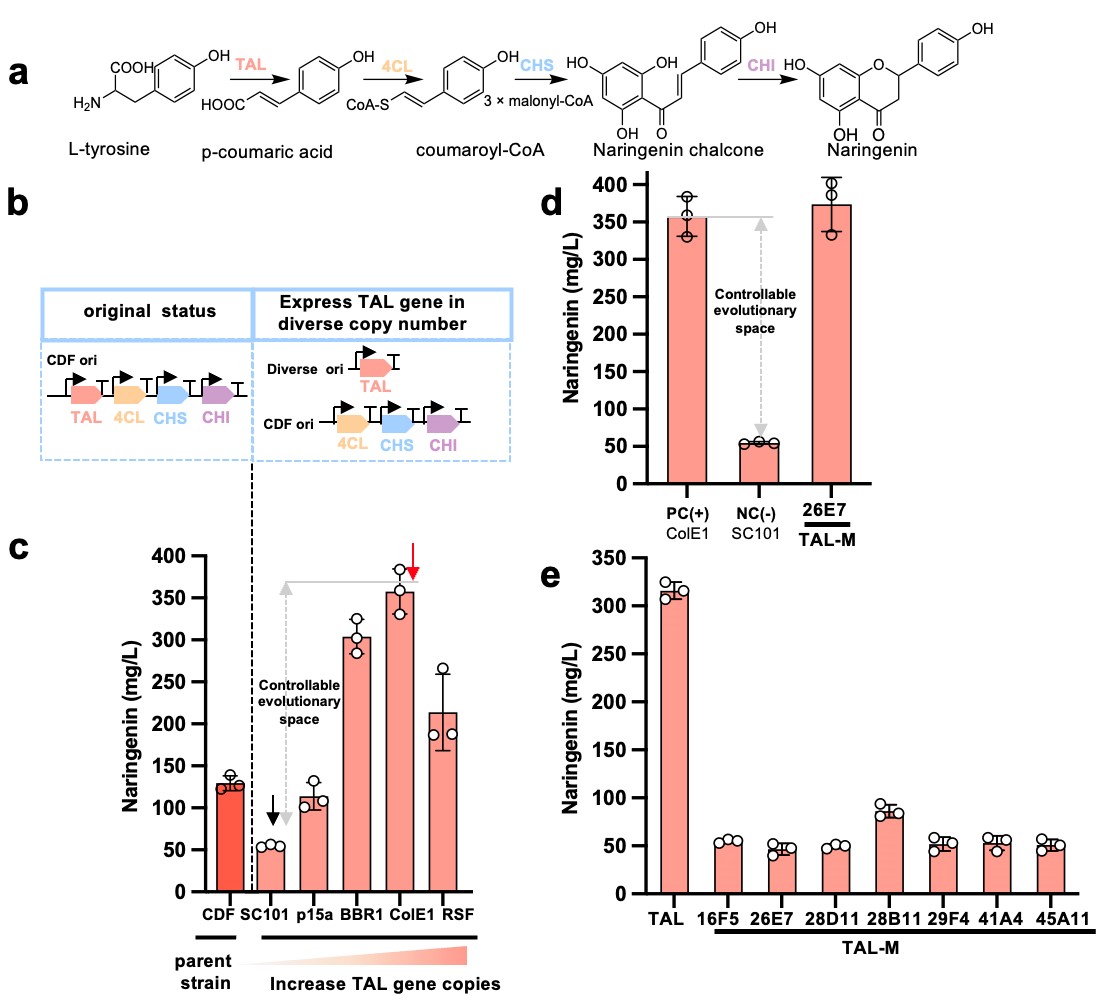
Figure 1. A predictable evolutionary trajectory and epistatic effect of the TAL gene. (Image by Prof. LOU)
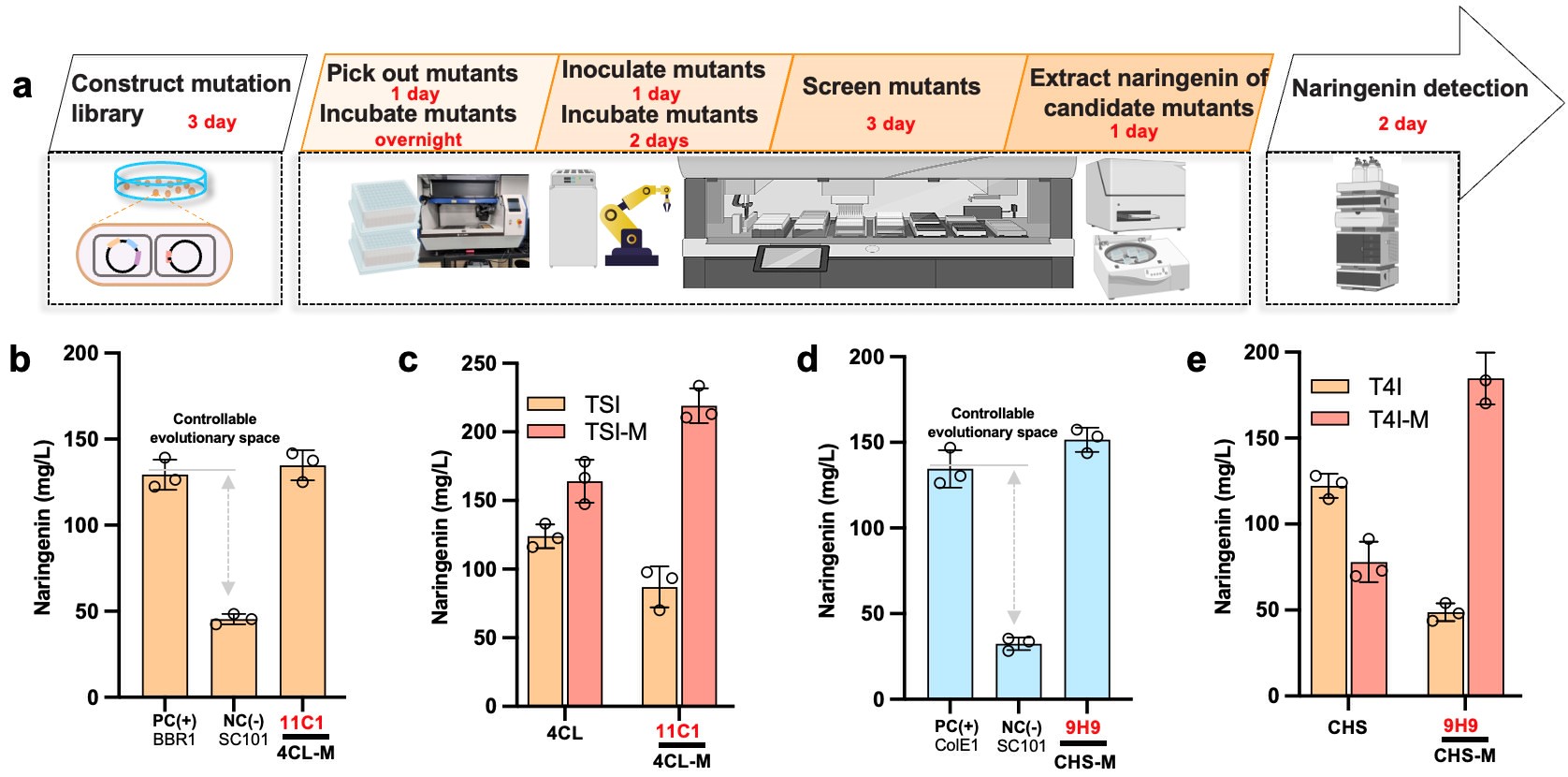
Figure 2. Biofoundry assists pathway debottlenecking along a predictable evolutionary trajectory and evaluates the gene epistatic effect. (Image by Prof. LOU)
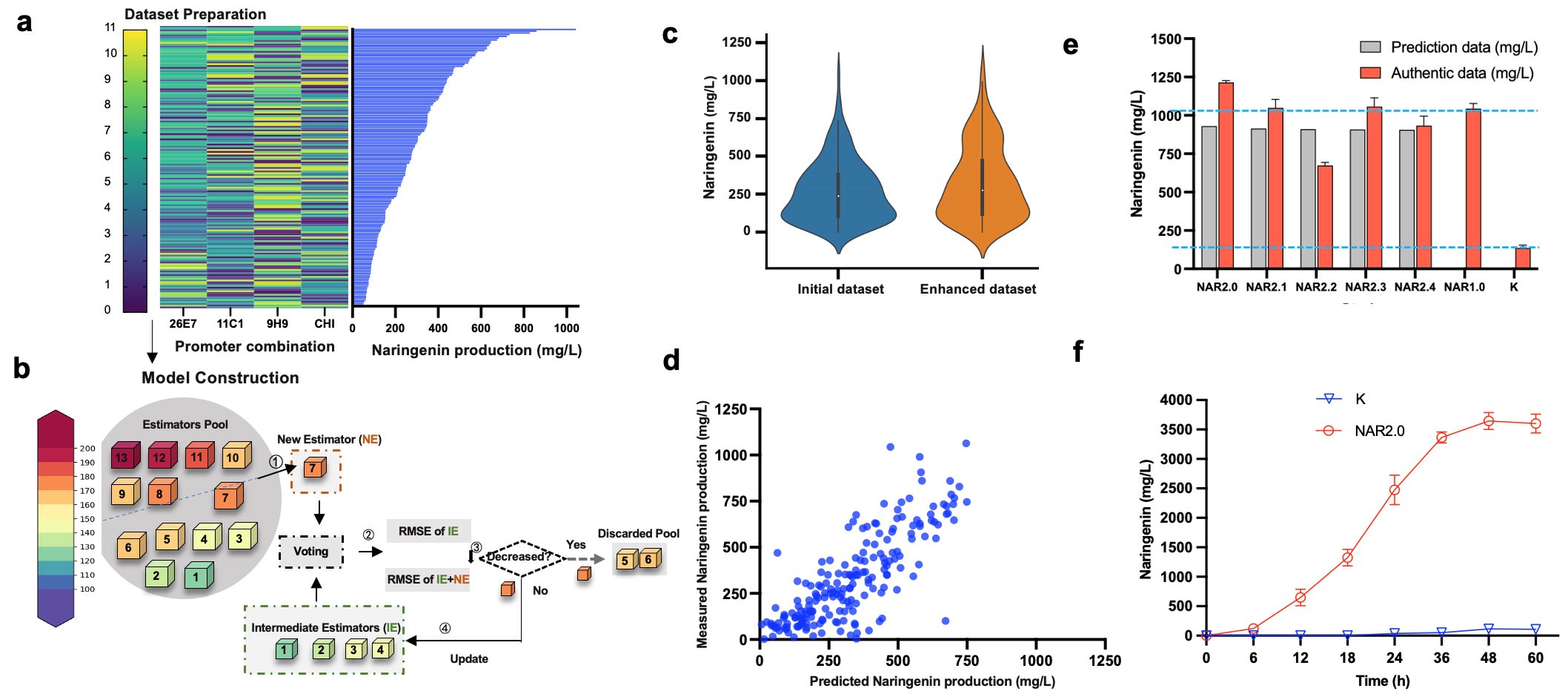
Figure 3. ProEnsemble framework further alleviates the epistatic effect of the evolutionary pathway. (Image by Prof. LOU)
Media Contact:
ZHANG Xiaomin
Email:xm.zhang@siat.ac.cn