Researchers Develop Novel Proteolysis-targeting (PROTAR) Live Attenuated Vaccine Strategies
Date:23-01-2025 | 【Print】 【close】
Recently, Prof. SI Longlong's team from the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences, published two studies in Nature Microbiology and Nature Chemical Biology.
Building on their 2022 introduction of the proteolysis-targeting (PROTAR) live attenuated influenza A vaccine (Nature Biotechnology 40, 2022: 1370-1377), these new studies detail the refinement and optimization of PROTAR vaccine strategies. The team constructed a library of live attenuated influenza A vaccines that utilize diverse E3 ubiquitin ligases to degrade viral proteins and achieve virus attenuation. To broaden the applicability of this approach, they advanced to the next-generation PROTAR 2.0 strategy.
Influenza continues to be a significant global public health concern, causing 3–5 million severe cases and 0.3–0.6 million deaths annually. Vaccination is widely considered as the most effective way to prevent influenza. Currently, the majority of licensed influenza vaccines are inactivated influenza vaccine (IIV) and cold-adapted live attenuated influenza vaccine (CAIV).
However, traditional vaccine strategies can result in the loss or incomplete matching of natural antigens from circulating influenza strains, potentially leading to reduced vaccine efficacy.
Converting the whole circulating virus into an attenuated, live vaccine could ensure a better antigen match and induce sufficient efficacy, offering a promising method for more effective vaccines. Using the cellular ubiquitin-proteasome system (UPS) to alter viral protein stability is a promising strategy for developing live-attenuated vaccines.
The team first engineered PROTAR influenza viruses to be attenuated by the ubiquitin–proteasome system, which degrades viral protein in conventional host cells but allows efficient replication in engineered cell lines for large-scale manufacturing (Fig. 1).
The team then constructed a PROTAR vaccine library by incorporating 22 distinct proteasome-targeting degrons (PTDs) into the C-terminus of the viral protein M1 via a conditionally cleavable linker, with each PTD being recognized by a different E3 ligases.
Depending on the PTD–E3 ligase pairs, PROTAR influenza viruses showed varying levels of attenuation in vitro. In animal models, the PROTAR vaccine candidates exhibited significant attenuated, induced broad-spectrum immune responses with varying intensity. They also provide robust cross-reactive protection against lethal infection by both homologous and heterologous viruses in a range of animal models, including adult mice, aged mice, mice with pre-existing flu immunity and ferrets.
Although the PROTAR vaccine approach shows potential for virus attenuation and vaccine design, the PTDs could only be incorporated at the terminal ends of viral proteins thus limiting its broad application.
The team finally developed the next-generation PROTAR vaccine approach, PROTAR vaccine 2.0, aiming to optimize and enhance the versatility of the PROTAR vaccine strategy.
PROTAR 2.0 enables the incorporation of PTDs at multiple sites within viral proteins, including the N-terminal, C-terminal and internal regions. The genome-wide investigation revealed that PROTAR 2.0 viruses, with two PTD-modified viral proteins, exhibited efficient replication in E3 ubiquitin ligase-knockout cells but were attenuated in conventional cells due to PTD-mediated proteasomal degradation. (Fig. 2).
In animal models (mice and ferrets), the PROTAR 2.0 viruses exhibit excellent safety characteristics. A single intranasal dose of PROTAR 2.0 virus vaccine induces robust humoral, mucosal and T cell immune responses, offering complete cross-reactive protection against both homologous and heterologous viral challenges.
Overall, these studies provide new approaches for developing safe and effective live attenuated vaccines.
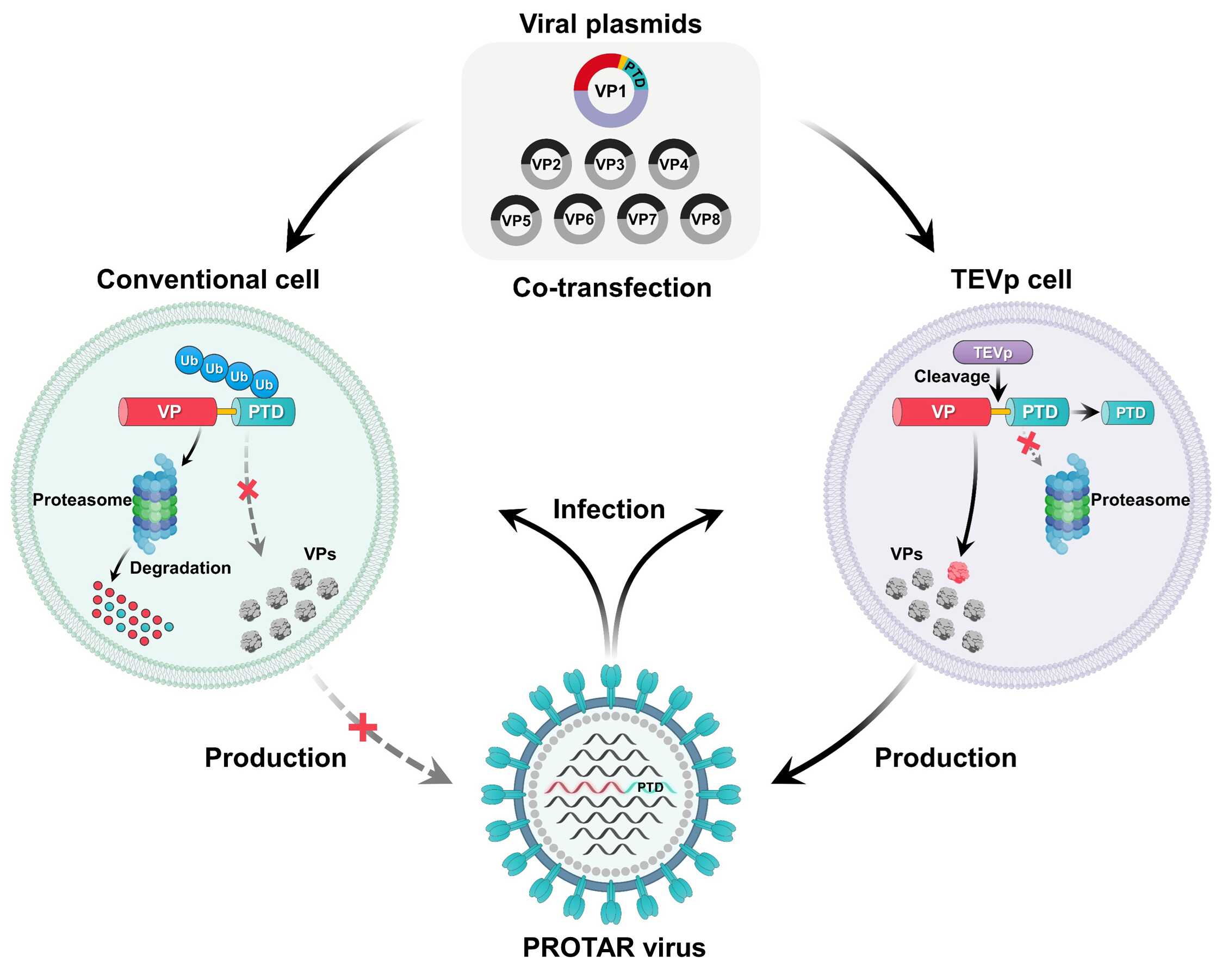
Fig.1: PROTAR viruses efficiently reproduce owing to TEVp-mediated viral protein stabilization in TEVp-expressing cells and are attenuated by proteasome-mediated viral protein degradation in conventional cells. VP, viral protein; Ub, ubiquitin. (Image by SIAT)
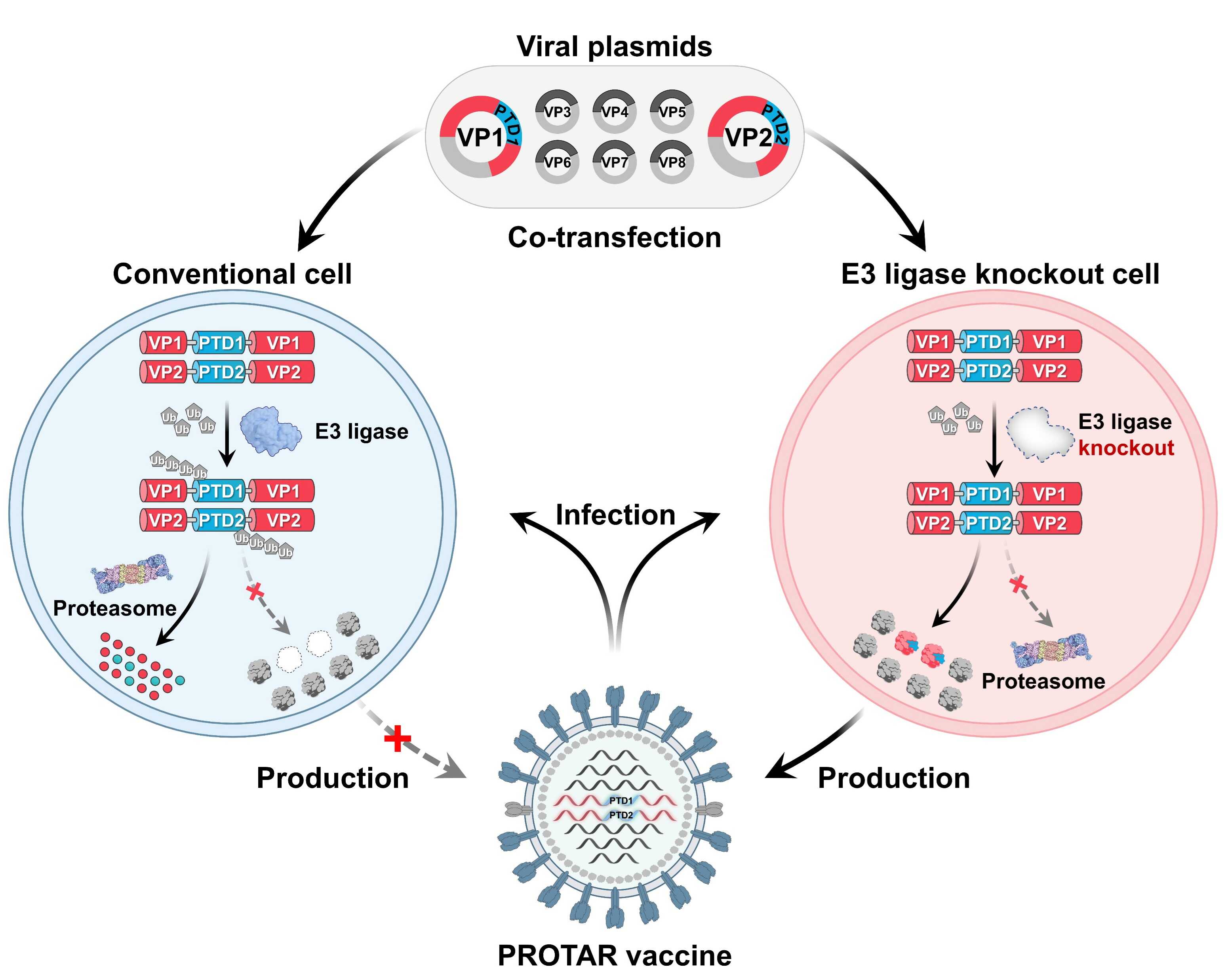
Fig. 2: PROTAR 2.0 viruses efficiently replicate in E3 ubiquitin ligase- knockout cells but are attenuated by E3 ubiquitin ligase-mediated proteasomal degradation of viral proteins in conventional cells. (Image by SIAT)
Media Contact: LU Qun
Email: qun.lu@siat.ac.cn
Download the attachment:
PROTAR Vaccine 2.0 generates influenza vaccines by degrading multiple viral proteins