Novel Neuron-like Ferroelectric Bioelectronics Enable Seamless Integration and Adaptive Communication with Neuronal Networks
Implantable bioelectronics are vital to neuroscience, neurological therapies, and brain-machine interfaces, serving as indispensable interfaces that enable communication between biological systems and external devices through the sensing, monitoring, and modulation of bioelectrical signals. However, conventional implantable bioelectronic devices struggle to integrate adaptively with neural tissues due to their lack of neuron-like structural and functional properties. This limitation affects both their performance and long-term reliability in integrating and communicating with neural tissues.
Recently, a research team led by Dr. DU Xuemin from the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences, has developed a novel neuron-like interface material and bioelectronic platform, termed ferroelectric bioelectronics (FerroE), which integrates neuron-like flexibility, surface topography, and functional behaviors into a single system, enabling highly integration and adaptive communication with neural systems.
This study was published in Advanced Materials on May 29.
The FerroE comprises three core components: biocompatible polydopamine-modified barium titanate nanoparticles, which enable efficient photo-to-thermal conversion and enhanced ferroelectric performance; a ferroelectric poly (vinylidene fluoride-co-trifluoroethylene) copolymer that generates real-time electric signals through reversible polarization changes; and cellular-scale micropyramid array structures that promote neuronal adhesion, neurite outgrowth, and interconnection.
By leveraging their synergistic interactions, the FerroE not only exhibit excellent flexibility, uniform topographical structures, highly efficient and stable light-induced polarization changes, and an outstanding ability to generate electric signals (producing approximately 3.6 V within 100 milliseconds, with no decay observed after approximately 10,000 cycles or 180 days under physiological conditions), but also enable seamless integration and adaptive communication with neuronal networks.
Dr. DU explained, "FerroE enables adaptive interfacing with both peripheral (vagus nerve) and central (motor cortex) neural networks in mice, allowing for wireless, non-genetic, and non-contact regulation of heart rate and motor behavior." Importantly, he noted, FerroE demonstrates a highly functionally stable and biocompatible interface with neurons, maintaining its performance for up to three months after in vivo implantation.
This study opens new directions for the development of next-generation neural interface materials and devices, potentially inspiring future advancements in adaptive brain-machine interfaces, tissue engineering, and biomedical technologies.
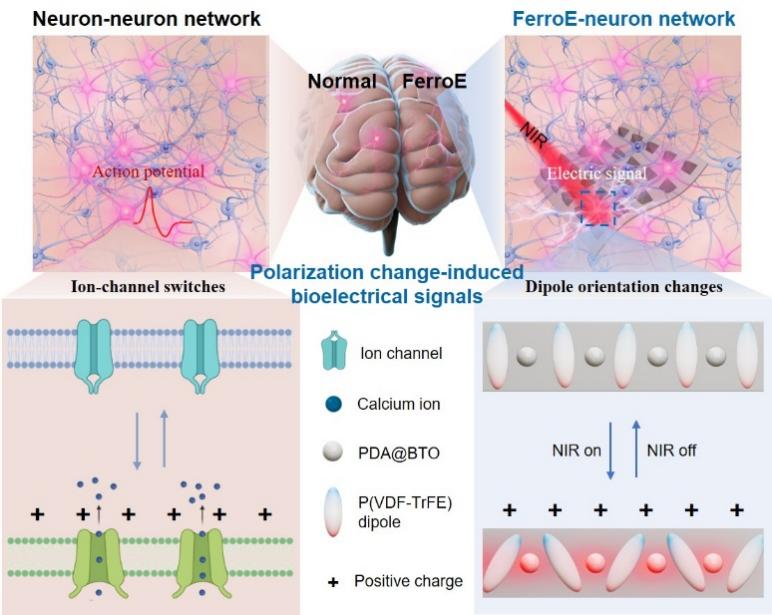
Conceptual scheme of neuron-inspired FerroE.(Image by SIAT)
File Download: