Deep Learning-based Model Enables Fast and Accurate Stroke Risk Prediction
Stroke is the second leading cause of death globally. Ischemic stroke, strongly linked to atherosclerotic plaques, requires accurate plaque and vessel wall segmentation and quantification for definitive diagnosis. However, conventional manual segmentation remains time-consuming and operator-dependent, whereas current computer-aided tools fall short in achieving the accuracy required for clinical applications. These technological bottlenecks severely hamper precise diagnosis and treatment of ischemic stroke.
In a study published in European Radiology, a research team led by Dr. ZHANG Na from the Shenzhen Institutes of Advanced Technology (SIAT) of Chinese Academy of Sciences, along with collaborators, has developed a fully learnable parameter based multi-task segmentation model and a structure-guided, two-stage small-target segmentation method based on high-resolution magnetic resonance (MR) vessel wall imaging. This approach enables automated and accurate segmentation and quantitative analysis of carotid arterial vessel lumens, vessel walls, and plaques, offering a reliable AI-assisted diagnostic tool for clinical risk assessment of ischemic stroke.
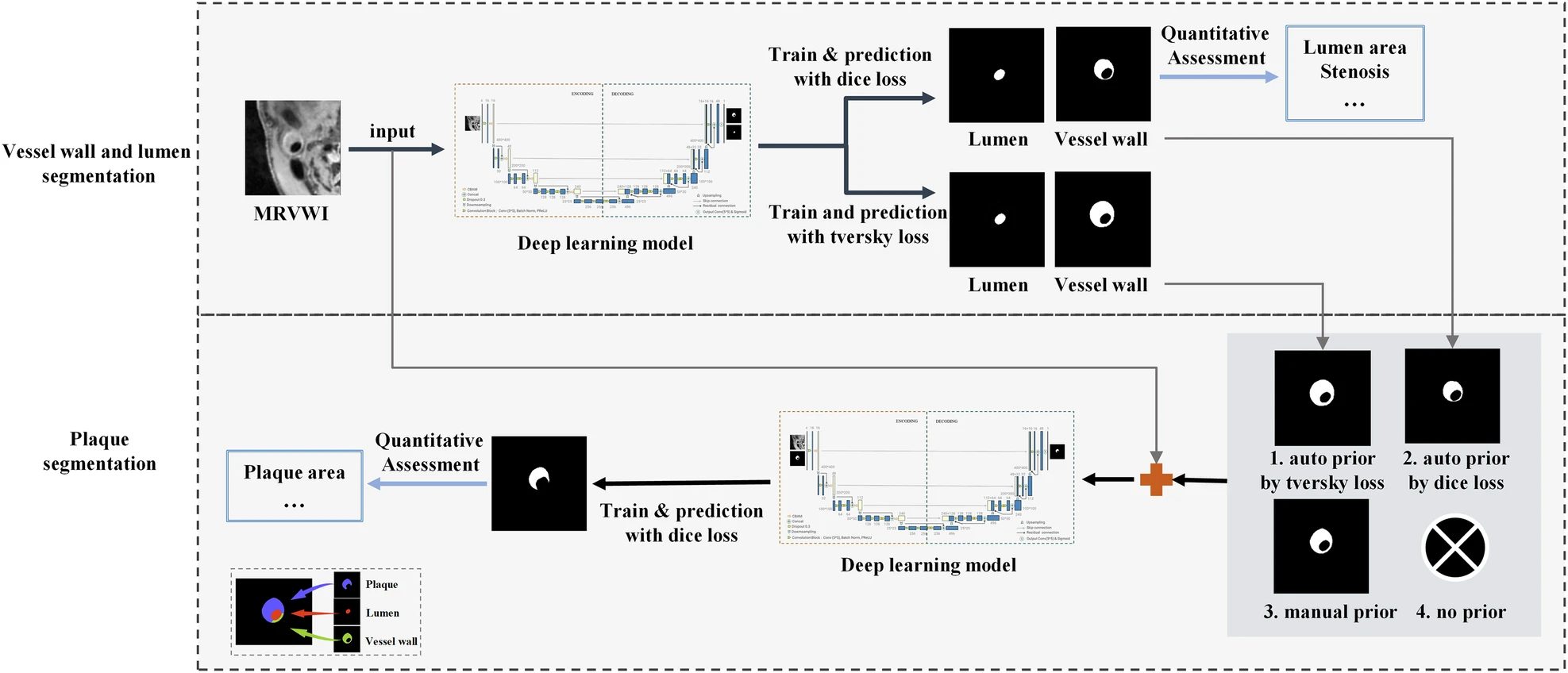
In this study, the proposed method consists of two key steps. The first step involves constructing a purely learning-based convolutional neural network (CNN), named Vessel-SegNet, to segment the lumen and vessel wall. The second step leverages vessel wall priors—specifically, manual priors and Tversky-loss-based automatic priors—to improve plaque segmentation by utilizing the morphological similarity between the vessel wall and atherosclerotic plaque.
This study included data from 193 patients with atherosclerotic plaque across five centers, all of whom underwent T1-weighted magnetic resonance imaging (MRI) scanning. The dataset was divided into three subsets: 107 patients for training and validation, 39 for internal testing, and 47 for external testing.
Experimental results demonstrated that most Dice similarity coefficients (DSC) for lumen and vessel wall segmentation exceeded 90%. The incorporation of vessel wall priors improved the DSC for plaque segmentation by over 10%, achieving 88.45%. Furthermore, compared to Dice-loss-based priors, Tversky-loss-based priors further enhanced the DSC by nearly 3%, reaching 82.84%.
In contrast to manual methods, the proposed technique provides accurate, automated plaque segmentation and completes quantitative plaque characteristic assessment for a single patient in under 3 seconds.
"The goal of our research is to leverage AI models to produce accurate, reproducible, and clinically relevant quantitative outcomes, which can assist healthcare professionals in stroke diagnosis and therapeutic decision-making," explained Dr. ZHANG.
Dr. ZHANG added, "In the future, we will need to conduct additional studies using other equipment, populations, and anatomical analyses to further validate the reliability of the research results."
Fig. 1: Overall technical schematic of the two-stage vessel walls and plaques segmentation and quantification method. (Image by SIAT)
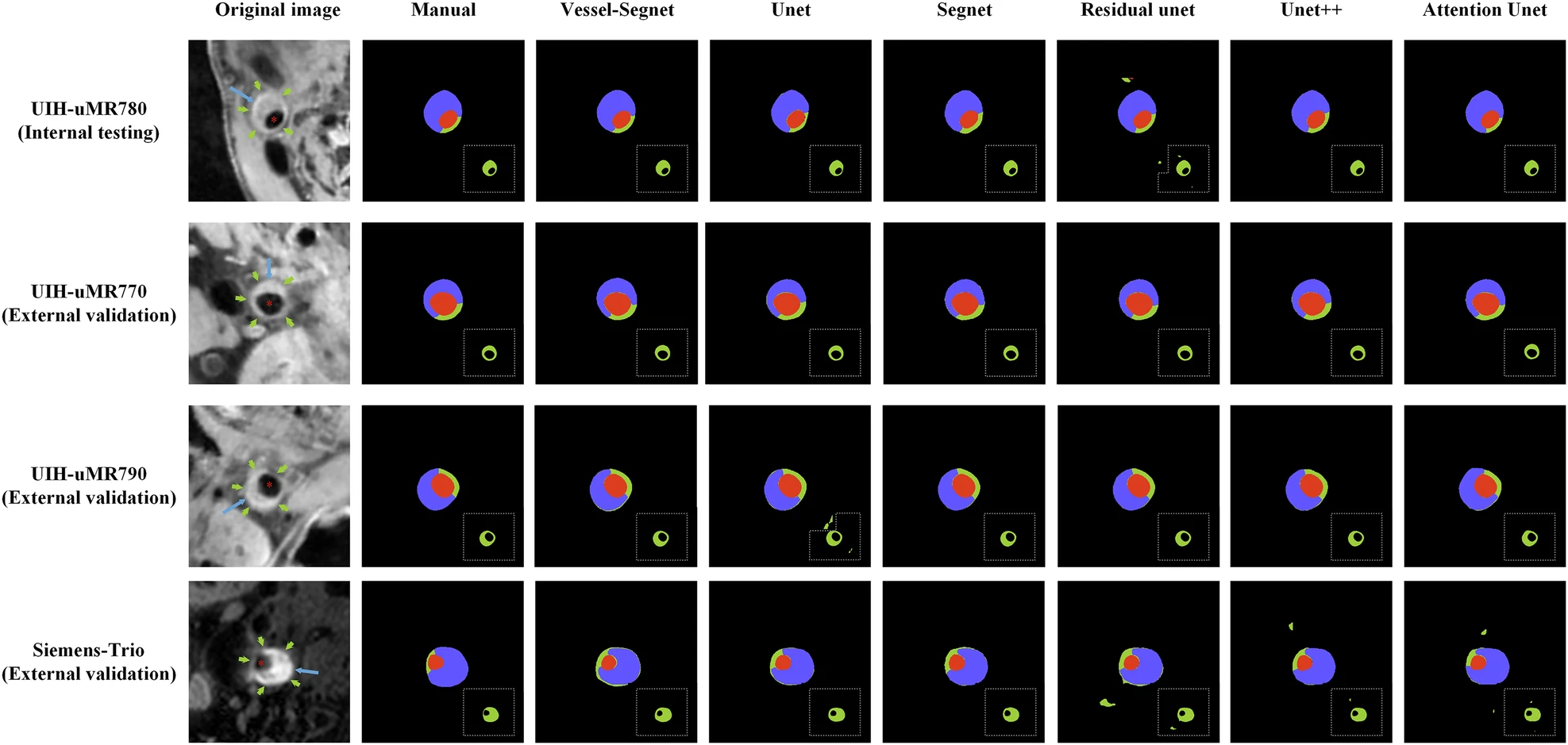
Fig. 2: Comparison of segmentation results for lumens, vessel walls, and plaques across different models. (Image by SIAT)
File Download: