
Artificial Ocean Carbon Recycling System Converts Seawater CO2 into Valuable Chemicals
Oceans absorb about one-third of atmospheric carbon dioxide (CO2) emissions, but harvesting this dissolved carbon from seawater for conversion into useful products remains a significant challenge. While direct ocean capture (DOC) of CO2 is emerging as a promising approach, no existing system has successfully integrated DOC with the direct conversion of CO2 into a valuable chemical feedstock.
In a study published in Nature Catalysis, a team led by GAO Xiang from the Shenzhen Institutes of Advanced Technology of the Chinese Academy of Sciences (CAS), in collaboration with XIA Chuan from the University of Electronic Science and Technology of China, developed an artificial ocean carbon recycling system that captures CO2 from seawater and directly converts it into succinic acid, a key monomer for the biodegradable plastic polybutylene succinate (PBS).
The proposed system combines electrochemistry with microbial fermentation in a unique cascade process.
Seawater is introduced into a five-chamber electrochemical reactor, where an applied electric field drives water splitting, generating protons that acidify the seawater in a targeted chamber. This pH shift efficiently converts dissolved carbonate species into gaseous CO2. The CO2 is then separated via a hollow-fiber membrane and delivered to a second reactor containing a custom-designed bismuth-based catalyst, which selectively reduces CO2 to formic acid. Finally, the formic acid is fed to an engineered strain of Vibrio natriegens for microbial fermentation, yielding succinic acid as the final product.
The system demonstrated exceptional stability and performance. In contrast to previous DOC devices, which typically operate for only a few hours, the team's five-chamber reactor continuously extracted CO2 from natural seawater sourced from Shenzhen Bay, China, for over 530 hours, achieving a carbon capture efficiency of 70%.
The estimated capture cost of approximately $230 per metric ton of CO2 is competitive with current state-of-the-art carbon capture technologies.
"This is the first demonstration that's going from ocean carbon dioxide all the way to a usable feedstock for bioplastic," commented XIANG Chengxiang, a specialist in chemical physics and materials science at the California Institute of Technology, is also cofounder and chief technology officer of the direct ocean capture company Captura, who was not involved in the work. He noted that the true focal point of the research is "taking that CO2 and turning it into a bioplastic monomer with promising stability and economics."
Furthermore, by incorporating different engineered microbes, the system can be tailored to produce a range of valuable industrial chemicals, such as lactic acid, alanine, and 1,4-butanediol, demonstrating the platform's versatility.
This study presents a sustainable strategy for upcycling ocean-derived CO2 and opens new avenues for electrochemically driven biochemical synthesis.
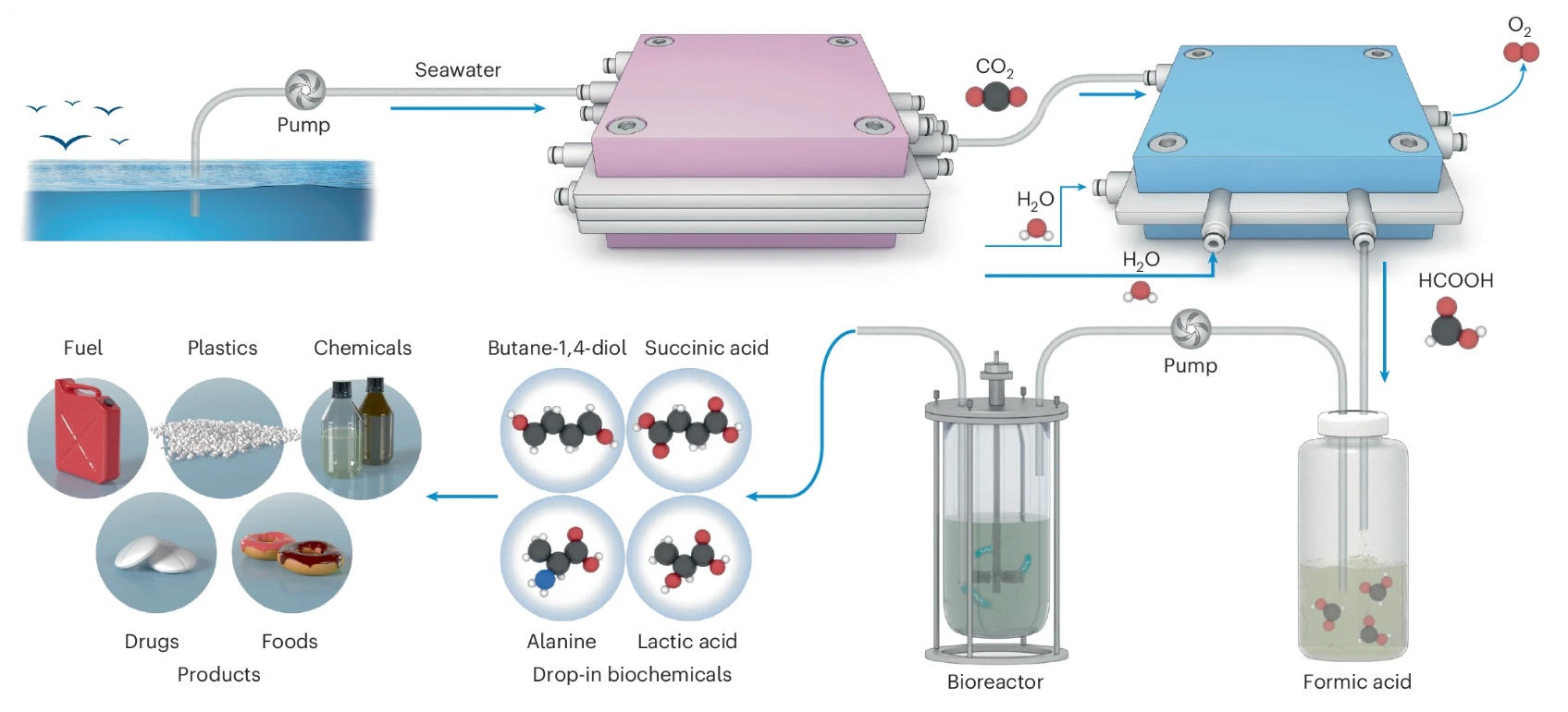
Schematic illustration of the artificial ocean carbon recycling system that captures and converts oceanic CO2 into drop-in biochemicals through decoupled electro-biocatalytic processes. (Image by SIAT)
File Download:

