Researchers Uncover the Mechanism of Plasma Membrane Remodeling that Drives Mitotic Cell Rounding
During cell division, adherent animal cells round up to create the precise spatial geometry required for accurate chromosome segregation, a process that depends on the coordinated remodeling of the cytoskeleton and plasma membrane. It is generally assumed that the membrane passively follows cytoskeletal reorganization during this process. However, whether the plasma membrane is subject to active regulation, and the precise mechanisms underlying the coordination between these two components, remain unclear.
In a study published in Journal of Cell Biology, a team led by Prof. LI Hongchang from the Shenzhen Institutes of Advanced Technology of the Chinese Academy of Sciences,uncovered a fundamental plasma membrane remodeling mechanism that governs the mitotic cell rounding.
In this study, researchers discovered that Numb, a protein known as a fate determinant in asymmetric cell division, undergoes significant phosphorylation at Ser7, Ser265, and Ser284 during symmetric mitosis. This phosphorylation is not mediated by the previously reported kinases aPKC or Plk1, but instead by Aurora A, a central regulator of mitosis progression.
Further investigation revealed that Numb phosphorylation is crucial not only for its proper localization at the plasma membrane but also for efficient membrane retraction during mitotic cell rounding. Overexpression of a non-phosphorylatable Numb mutant, which remains stably localized at the membrane, significantly disrupts membrane retraction in mitotic cell.
Additional analyses demonstrated that impaired Numb phosphorylation disrupts mitotic cell rounding and leads to defects in spindle orientation and chromosome segregation.
To elucidate the molecular mechanism linking Numb phosphorylation to membrane remodeling, researchers employed mass spectrometry, co-immunoprecipitation, and actin cortex binding assays. They found that Numb directly binds to myosin I, a key family of motor proteins that link the plasma membrane to the actin cortex. This interaction prevents myosin I from binding to F-actin, thereby weakening the membrane-cytoskeleton linkage. At the onset of mitosis, Aurora A precisely reverses this state by phosphorylating Numb, triggering its dissociation from the plasma membrane. Consequently, myosin I is released and free to rebind the actin cortex. This coordinated molecular switch enables synchronized retraction of the plasma membrane and remodeling of the cytoskeleton, facilitating efficient mitotic cell rounding.
Moreover, the study demonstrated that Numb phosphorylation regulates cell shape during protease-induced rounding, underscoring the broad relevance of this mechanism across diverse cellular processes involving morphological remodeling.
"Our work offers novel insights into the mechanisms of membrane remodeling in cell division and may provide potential strategies for mitosis-targeted cancer therapies," said Prof. LI.
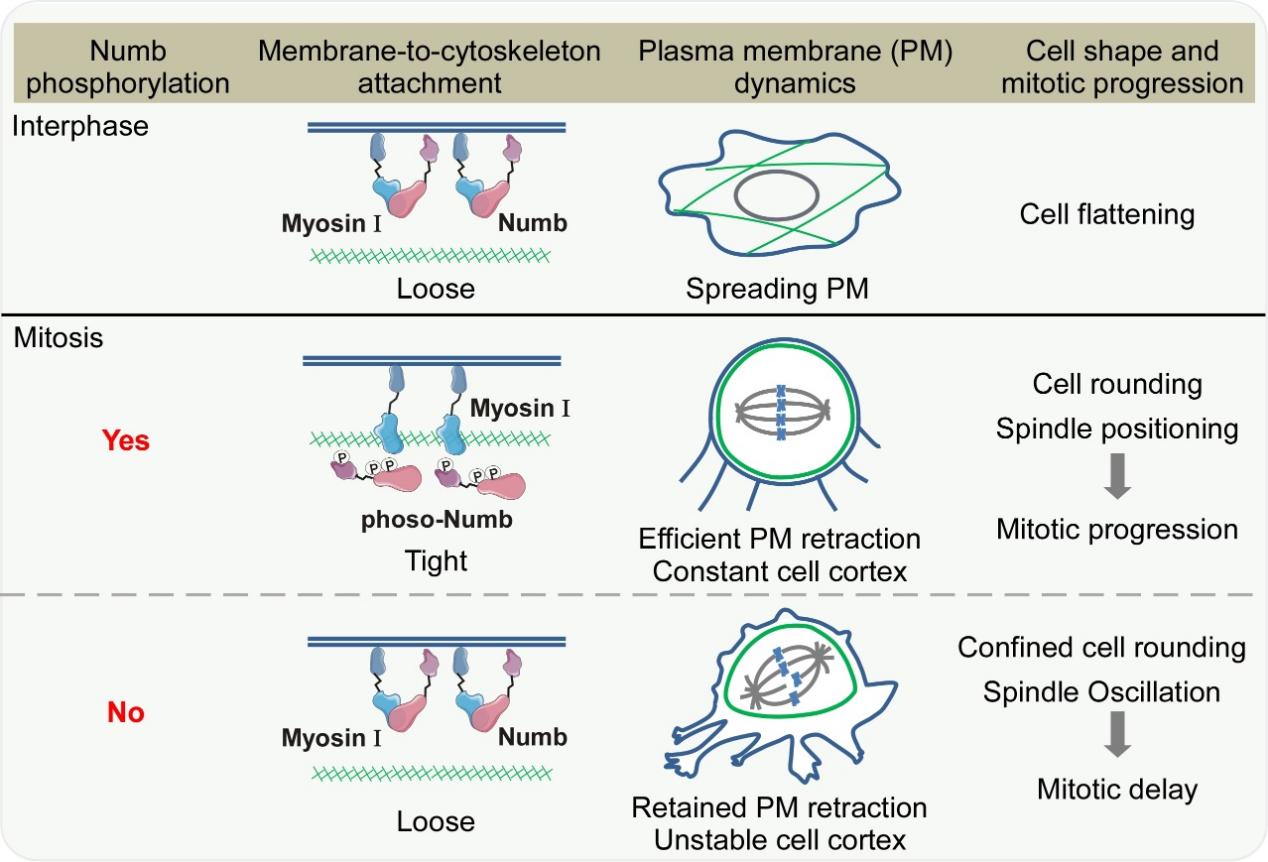
A model to illustrate the mechanism and function of Aurora A phosphorylation of Numb in membrane retraction, cell rounding as well as mitotic progression through the modulation of Myosin I family proteins in mitosis. (Image by SIAT)
File Download: