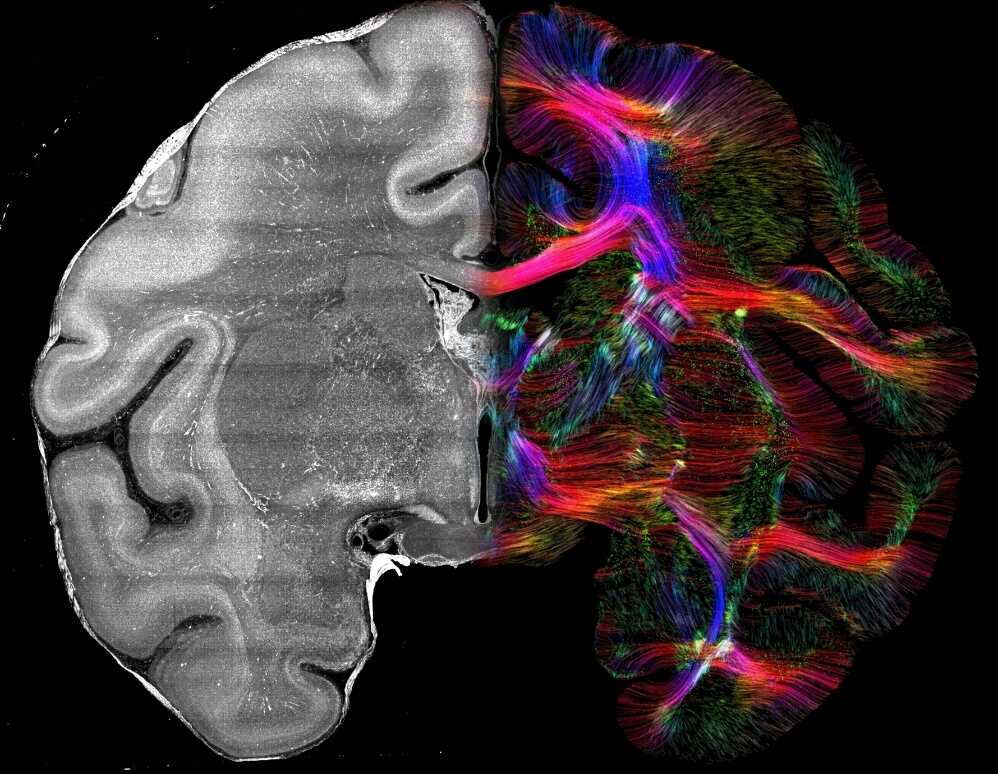
Whole-Body PET/CT Study Maps Inter-Organ Metabolic Networks Across the Glycemic Spectrum
Diabetes is now understood to be a systemic metabolic disorder, involving disruptions across multiple organs rather than a single glycemic pathway. Yet, in clinical practice, metabolic health is still assessed mainly through blood glucose and HbA1c, markers that capture only global outcomes, not the underlying coordination among organs. What remains largely invisible is how the brain, liver, pancreas, muscle, adipose tissue, and other organs interact as an integrated metabolic network. There is therefore a pressing need for a method that can map inter-organ metabolic coordination in vivo, quantify how this coordination differs across glycemic states, and identify early, network-level signatures of metabolic imbalance.
Recently, a research team led by Prof. HU Zhanli from the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, in collaboration with Prof. DONG Mengjie from Peking University Shenzhen Hospital, uncovered how metabolic coordination among major organs shifts across normal glycemia, prediabetes, and diabetes. The work was published in European Journal of Nuclear Medicine and Molecular Imaging in November 2025.
Using a multi-center cohort of 1,149 participants spanning normal glycemia, prediabetes, and diabetes, the team examined how metabolic coordination among organs, the "inter-organ metabolic network", differs across glycemic states. Instead of analyzing each organ in isolation, the researchers quantified how strongly organs such as the brain, liver, pancreas, and muscle relate to one another in their metabolic activity, and how each individual’s pattern deviates from a healthy reference.
The analysis revealed a clear trend. As glycemic status shifts from normal to prediabetes and diabetes, the coordination between organs becomes weaker, with fewer and less stable metabolic links. Early changes were most evident in brain-centered connections, especially those linking the brain with the liver, pancreas, and skeletal muscle, suggesting that the neuro-peripheral metabolic axis is particularly sensitive to early metabolic imbalance. At the individual level, people with prediabetes showed more diverse metabolic patterns, whereas those with diabetes exhibited more concentrated but deeper disruptions in specific organ-to-organ relationships. Machine-learning interpretation further confirmed that these brain-peripheral connections were the strongest indicators of metabolic state, highlighting their central role in systemic metabolic regulation.
This study introduces a systems-level view of metabolic health by focusing on how organs coordinate with one another rather than on glucose levels alone. The results show that disruptions in this coordination appear earlier than traditional clinical markers, suggesting that whole-body PET/CT could help identify metabolic risk at a much earlier stage. The network signatures uncovered here also offer a basis for more personalized metabolic profiling. The team notes that future work will aim to validate these findings in longitudinal cohorts and explore whether early interventions can help restore disturbed organ-to-organ coordination.
Prof. Zhanli Hu (SIAT) and Prof. Mengjie Dong (Peking University Shenzhen Hospital) are the corresponding authors. Dr. Xuetong Tao (postdoctoral researcher at SIAT) is the first author.This work was supported by the National Natural Science Foundation of China, the National Key R&D Program, the Guangdong Natural Science Foundation, and the Shenzhen Science and Technology Innovation Commission.
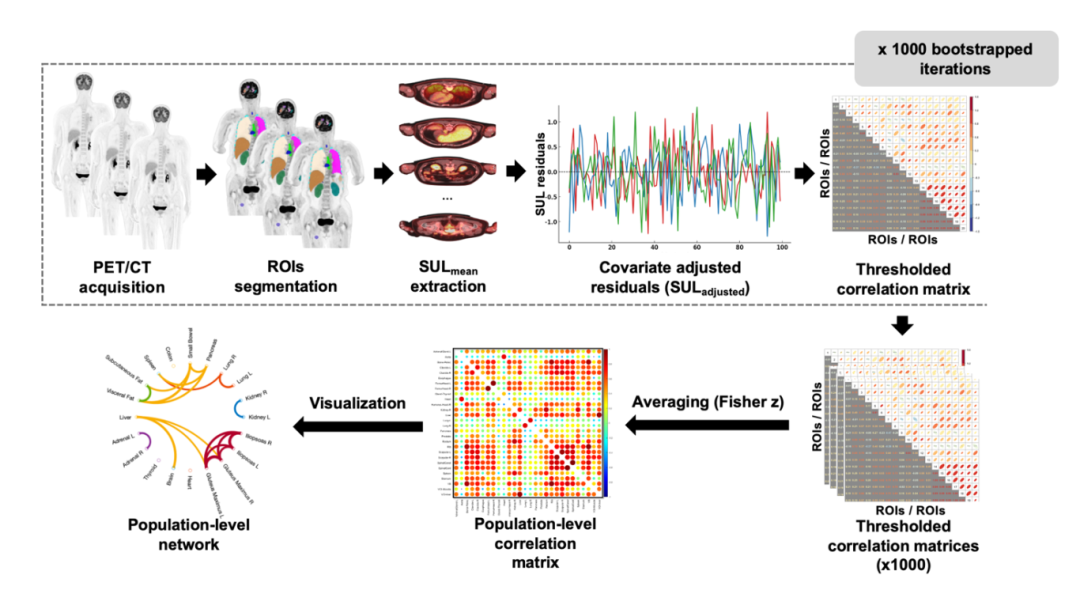
Workflow for constructing population-level inter-organ metabolic networks using whole-body [¹⁸F] FDG PET/CT. (Image by SIAT)
File Download: