Target binding of black phosphorus nanomaterial to polo-like kinase 1 for cancer chemotherapy: a mutual selection of nanomaterial and protein
Understanding how nanomaterials interact with biological macromolecules is essential for advancing nanomedicine. Over the past two decades, the "protein corona" concept has guided research into nanoparticle protein interactions, yet most studies have focused on nonspecific adsorption determined mainly by the physicochemical properties of nanomaterials. Whether nanomaterials can specifically recognize proteins and the mechanisms underlying such selectivity remain largely unknown.
A research team led by Prof. LI Yang from the Shenzhen Institutes of Advanced Technology (SIAT), Chinese Academy of Sciences, uncovered how black phosphorus nanomaterials (BPNMs) recognize and bind a key cell cycle regulator, polo like kinase 1 (PLK1). This work, entitled "Target binding of black phosphorus nanomaterial to polo like kinase 1 for cancer chemotherapy: a mutual selection of nanomaterial and protein," was recently published in Exploration.
The researchers found that BPNMs uniquely and selectively interact with PLK1, while showing little or no affinity for other members of the PLK family. This selective binding causes PLK1 molecules to aggregate, suppresses their enzymatic activity, and interferes with the division of cancer cells.By Using nanobiological, biochemical, and computational simulation approaches, the team systematically compared how BPNMs interact with five homologous PLK proteins (PLK1 to PLK5). They discovered that the strong preference for PLK1 arises from its distinctive physicochemical features, including its positively charged surface regions, hydrophobic patches, and tightly folded secondary structure.
Further structural mapping revealed that BPNMs interact simultaneously with two key functional domains of PLK1, the kinase domain responsible for enzyme activation and the polo box domain that controls protein degradation. By targeting both domains, BPNMs block PLK1 activation and degradation, leading to complete inhibition of its function in cell cycle regulation.
To overcome the instability and oxidation sensitivity of black phosphorus, the researchers developed cell membrane coated BPNMs that showed greatly improved stability, prolonged circulation, and enhanced tumor targeting. In animal models, these biomimetic nanomaterials achieved potent antitumor efficacy and excellent biocompatibility after systemic administration.
This study introduces the concept of "mutual selection" between nanomaterials and proteins, showing that their interactions are jointly defined by the physicochemical properties of both sides. The discovery provides new mechanistic insight into nano bio recognition and establishes a theoretical foundation for the rational design of targeted, safe, and effective nanomedicines.
This work was jointly led by Prof. LI Yang and Associate Prof. ZHANG Guofang from SIAT. Dr.LIU Fangfang, Dr. LI Zhongda, and Mr. ZENG Yanqiao are co first authors. The research was supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China, the Guangdong Provincial Natural Science Foundation, and the Shenzhen Science and Technology Innovation Commission. The research was carried out in collaboration with Prof. YU Xuefeng and Associate Prof. GENG Shengyong, both of are co authors of the study.
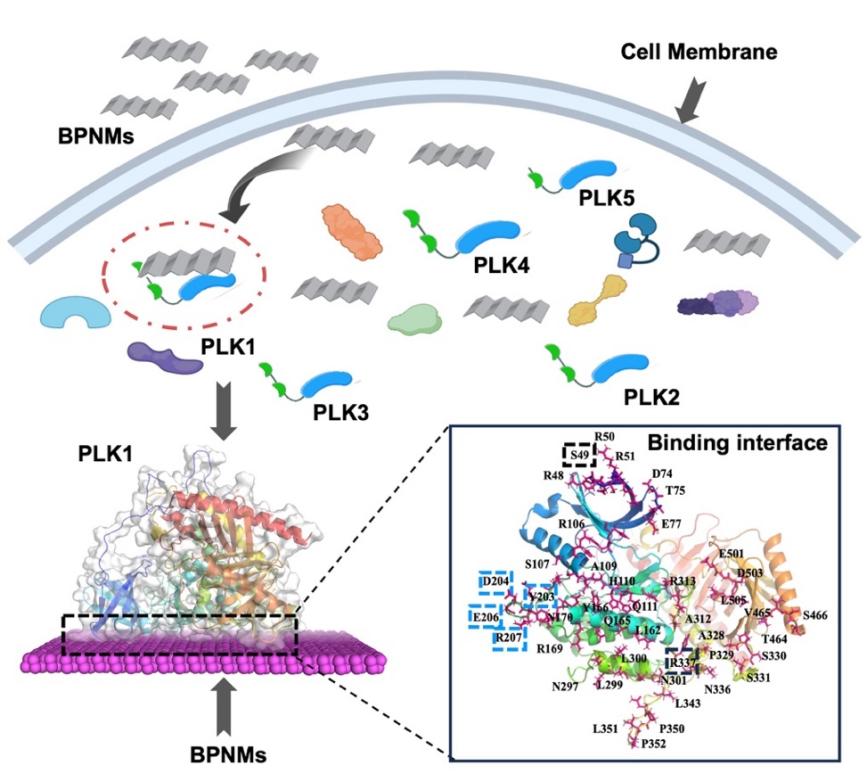
BPNMs selectively recognize PLK1 among the PLK family through a distinct dual-domain binding interface.(Image by SIAT)
File Download: