
Bacteroides fragilis Activates a Cholinergic Gut–Brain Circuit to Suppress Seizures
In a study published in Neuron on January 16, a research team led by Prof. LIU Xin'an and Prof. CHEN Zuxin from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences revealed how Bacteroides fragilis (B. fragilis) activates a novel cholinergic gut-vagus-brain circuit, and demonstrated its therapeutic potential in both animal models and children with refractory epilepsy.
Epilepsy is a chronic noncommunicable disease of the brain that affects around 50 million people worldwide. Nearly one-third of patients experience recurrent seizures even with medications. Antiepileptic drugs often come with significant side effects. To develop safer and more targeted therapies, the gut-brain axis has become an emerging area. However, it remains a question that how do specific gut microbes shape neural excitability through precise molecular and neural pathways.
In this study, researchers observed that the children with epilepsy exhibited a marked reduction in B. fragilis, and they confirmed this finding by analyzing microbiome profiles from children with refractory epilepsy. In mouse models of pentylenetetrazole- and kainic acid-induced seizures, researchers found that oral administration of B. fragilis substantially reduced both seizure frequency and severity, establishing that the supplement of B. fragilis can reshape brain excitability.
Through molecular analyses, cell-type-specific profiling, neural circuit tracing, and functional manipulation, researchers found that B. fragilis selectively activated choline acetyltransferase-positive epithelial cells in the colon. These cells released acetylcholine, which strengthened vagal sensory signaling to the brainstem. Enhanced vagal transmission subsequently modulated neuronal activity in nucleus tractus solitarius and hippocampal circuits, leading to reduced seizure susceptibility.
Moreover, researchers found that co-enrichment of Lactobacillus was associated with increased production of metabolites such as succinate, acetate and nicotinate, which potentiated colonic cholinergic signal. In mice, this microbial synergy significantly enhanced antiseizure effects. In children receiving B. fragilis therapy, clinical responsiveness was corelated with higher levels of Lactobacillus colonization, suggesting that microbial co-colonization patterns may shape the efficacy of probiotic-based therapies.
A randomized trial was conducted in pediatric patients with drug-resistant epilepsy. Oral B. fragilis supplementation demonstrated significant antiseizure effects, which was consistent with the mechanisms identified in animal studies and confirmed the microbe’s therapeutic potential in clinical settings. The findings identified B. fragilis as one of the probiotic candidates for epilepsy intervention
This study reveals that a specific microbial species activates a cholinergic epithelial-vagal-brain circuit to regulate neural excitability. And it demonstrates how microbial ecology can shape therapeutic outcomes, providing a foundation for microbiota-based interventions for neurological disorders such as refractory epilepsy.
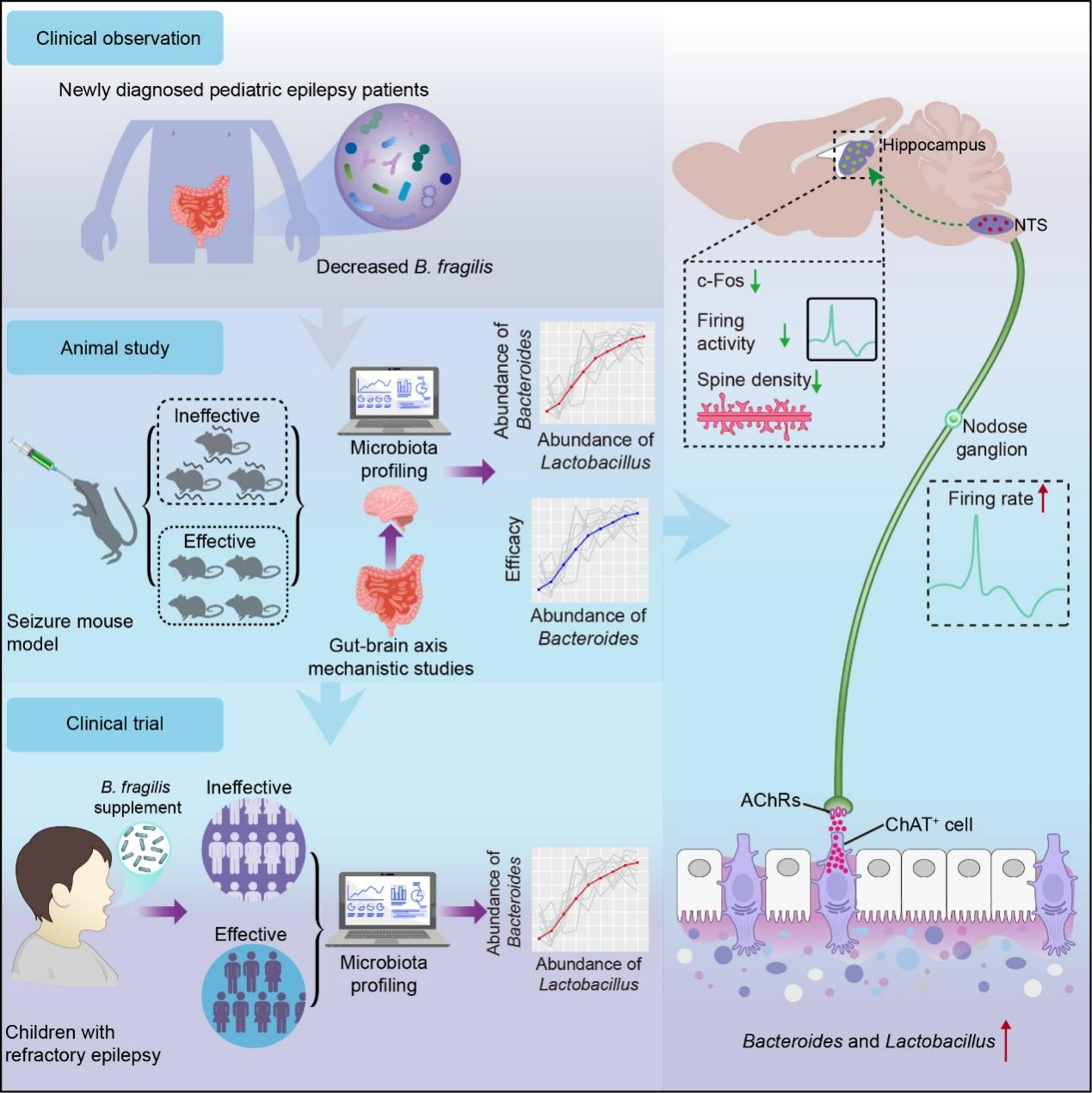
Antiseizure effects of Bacteroides fragilis are mediated by gut–vagus–brain cholinergic signaling and reinforced by Lactobacillus, with clinical validation in pediatric refractory epilepsy. (Image by SIAT)
File Download: