CEST-MRI Study Develops Joint Motion–Intensity Correction Framework for Enhanced Quantitative Imaging
Chemical Exchange Saturation Transfer (CEST) MRI is a molecular imaging approach that can sensitively probe tissue metabolism and microenvironment through frequency-selective saturation and exchange. Yet, in practical analysis, this information is only reliable if signals across multiple saturation frequency offsets are aligned before constructing Z-spectra and parametric maps.
What remains largely unaddressed is that CEST images move and change intensity simultaneously, as anatomy shifts and frequency-dependent contrast evolves during acquisition. Even subtle misalignment or mis-modeled intensity can warp the Z-spectrum and corrupt voxel-wise biochemical indices such as amide proton transfer and creatine, underscoring the need for a unified framework that jointly corrects motion and intensity.
In a study published in IEEE Journal of Biomedical and Health Informatics, a research team led by Prof. HU Zhanli and WU Yin from the Shenzhen Institutes of Advanced Technology of the Chinese Academy of Sciences, and Prof. LUO Dehong from the Cancer Hospital Chinese Academy of Medical Sciences, Shenzhen, developed the motion–intensity joint correction framework specifically tailored to CEST-MRI.
The researchers built on finite-element digital image correlation to cast CEST-MRI registration as a mechanically constrained problem, enforcing spatially and temporally coherent displacement fields across the dynamic series. They then alternated this physics-informed motion estimation with adaptive global intensity correction, explicitly decoupling motion from frequency-dependent intensity changes and providing a cleaner foundation for subsequent Z-spectrum construction and quantitative CEST mapping.
The researchers applied the framework to 48 CEST-MRI datasets, including simulated liver, healthy volunteer brain, and ex vivo Bama pig heart scans with varying motion amplitudes, to test its performance across organs and motion conditions. Compared with conventional and deep learning–based registration, the proposed approach better preserved fine anatomical structures, stabilized frame-to-frame dynamics, and produced more coherent CEST series overall, with clear advantages under larger or non-uniform motion.
At the parametric level, the jointly corrected data yielded amide proton transfer and creatine maps that were more compact and physiologically coherent, with fewer artificial hotspots and reduced frame-to-frame fluctuations. Further regional analysis confirmed that these stabilized CEST readouts were more sensitive to subtle tissue differences, providing a stronger imaging marker of underlying metabolic state.
This study introduces a dynamics-aware view of CEST-MRI by treating it as a mechanically constrained, contrast-evolving system, and shows that jointly correcting motion and intensity yields a more stable basis for quantitative biochemical mapping, potentially revealing subtle metabolic perturbations that conventional processing fails to detect.
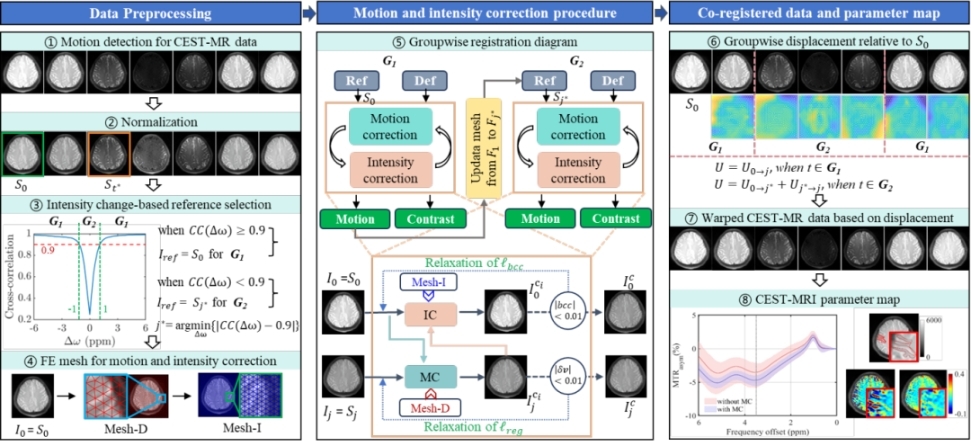
The overall registration framework based on staggered motion and intensity correction process. (Image by SIAT)
File Download: